Introduction
Aromatic hydrocarbons or arenes can be defined as cyclic hydrocarbons with delocalized pi-electrons between carbon atoms of ring. The phenomenon of exhibit aromatic nature is called as aromaticity.
Since benzene is simplest aromatic compound therefore aromatic hydrocarbons can be defined as hydrocarbons which contain one or more benzene rings. They are also well known for their strong, pungent aromas.

Task
The organic compounds which are composed of carbon and hydrogen are called as hydrocarbons. Hydrocarbons are most common organic compounds and exhibit versatile applications. This is due to presence of carbon atom as backbone in these compounds. We know that carbon shows tetra-valency and can form maximum four covalent bonds with same or other elements. The tendency of formation of covalent bonds with same atom results the formation of ling chain compounds and this is called as catenation.
Hydrocarbons also have a long chain of carbon atoms in the form of straight, branched or cyclic forms. On the basis of structure, they can be classified as aliphatic and cyclic hydrocarbons. Aliphatic hydrocarbons are straight compounds with single or multiple covalent bonds. On the basis of single or multiple covalent bonds, aliphatic compounds can be alkanes, alkenes and alkynes. Alkanes have only single covalent bonds but alkenes and alkynes have multiple covalent bonds. Alkenes have double covalent bonds and alkynes have triple covalent bonds between carbon atoms of parent chain.
Cyclic compounds have ring structure which can be alicyclic and aromatic in nature. Alicyclic compounds are aliphatic cyclic compounds with one or more ring formations. They can be saturated or unsaturated in nature with aliphatic side chains. They are not aromatic and exhibit properties like aliphatic compounds.
Aromatic compounds are also cyclic compounds with conjugated bonds which provide them stability and also influence their chemical and physical properties. Benzene is simplest organic compound with hexagonal planer structure. It contains three double bonds placed in three double bonds alternate manner. The presence of alternate double bonds causes resonance in molecule. Each carbon atom of benzene is sp2 hybridized that makes the molecule planer. One unhybridized p-orbital at each carbon atom involves in pi-bond formation with neighbor carbon atom. Therefore three pi-bonds or six pi-electrons remains delocalized over six carbon atoms. It makes molecule stable and aromatic in nature.
Petroleum and coal tar are major sources of aromatic compounds. They are well known for their unique chemical and physical properties. Aromatic compounds with more than one benzene ring are called as poly-aromatic hydrocarbons. They involve in atmospheric pollution and are known carcinogenic in nature. Aromatic compounds are also precursors to nucleotides and amino acids. They are non-polar hydrocarbons which are insoluble in water as there is no condition to form ions or H-bonds with water molecules. Due to extra stability, they are usually un-reactive and widely used as inert solvent for many organic and inorganic reactions. They burn with sooty yellow flame.
We know that hydrocarbons with multiple bonds are unsaturated in nature lie alkenes and alkynes. Due to this un-saturation, they tend to give addition reactions. Unlike unsaturated hydrocarbons, aromatic hydrocarbons are stable due to resonance and give characteristic electrophilic substitution reactions. In these reactions the carbon ring acts as a nucleophile and an electrophile attack on benzene ring to form a substituted product. Since one of the H-atom of ring is substituted with coming electrophile so product also retains its stability and aromatic in nature. On the contrary, in addition reactions, aromatic compound may lose their aromaticity so does not prefer to give such reactions.
Benzene is simplest and smallest aromatic compound with six carbon atoms. Here these six carbon atoms are arranged in hexagonal manner. It contains three pi-bonds which are arranged in alternate manner and make it aromatic and stable hydrocarbon. Other aromatic hydrocarbons are made from benzene only. So we can say that all aromatic hydrocarbons must have benzene ring. For example, in naphthalene, there are 2 benzene rings fused with each other with 2 common carbon atoms. Similarly any side chain on the aromatic ring results different aromatic compounds such as phenol, anisole, aniline etc.

Aromatic compounds exhibit unique chemical and physical properties due to their extra stability. Aromatic hydrocarbons have wide applications in several industries. For example, toluene is used as the solvent for model glues whereas naphthalene is used as mothballs. Phenanthrene is an intermediate product of different synthetic process for the manufacturing of dyes, explosives, and drugs. Another important aromatic compound is Trinitrotoluene (TNT) or 2, 4, 6-trinitrotoluene which is mainly used as an explosive and for the preparation of other explosives. Pyrocatechol or 1, 2-benzenediol is marketed as catechol which is one of the key component of photographic developer.
Polycyclic aromatic hydrocarbons are aromatic compounds with more than one aromatic benzene ring. They are also called as polynuclear aromatic hydrocarbons. In these aromatic compounds, two or more aromatic rings are fused together and share two or more carbon atoms. For example, Naphthalene is formed from two benzene rings and is smallest polycyclic aromatic hydrocarbon.
With change in position of aromatic rings, the molecule also gets change. For example, Anthracene and Phenenthrene both are composed of three aromatic rings with different positions. It affects the chemical and physical properties of them. As the molecular weight increases, the resistance towards oxidation- reduction and vapourisation also increases for polyaromatic hydrocarbons. On the basis of molecular weight, they can be classified as low molecular weight and high molecular weight compounds. The lower molecular weight polycyclic aromatic hydrocarbons contain 2 to 3 ring group like naphthalenes, fluorenes, phenanthrenes, and anthracenes. They are toxic compounds for aquatic organisms. High molecular weight polycyclic aromatic hydrocarbons contain 4 to 7 ring such as chrysenes, coronenes etc. They are carcinogenic in nature. In the IUPAC (International Union of Pure and Applied Chemistry) naming of polycyclic aromatic hydrocarbons structure diagram should contain the greatest possible number of rings is in a horizontal row. The orientation must be in such a way that maximal numbers of rings are placed in the upper right quadrant and the minimal number in the lower left quadrant.
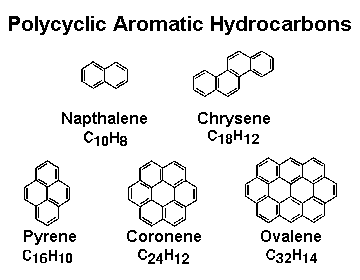
Process
In a following test there will be 10 questions by which you can improve yourself! Good luck!

https://play.kahoot.it/#/k/07b2f158-5914-4653-8794-35c3d8e5f83a
Evaluation
|
Correct answers |
Level of knowladge |
|
1-3 |
Very poor, read through again and try once more! |
|
4-5 |
Below average, find out your mistakes and try again! |
|
6-7 |
Good/average, but you can do better! |
|
7-9 |
Very good/ above average, you have done good job! |
|
10 |
Excellent! Thank you! |
Conclusion
To learn more check out these:
- https://www.youtube.com/watch?v=kXFEex-dABU
- http://www.chemguide.co.uk/organicprops/arenes/background.html
- https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/organic-chemistry-23/aromatic-hydrocarbons-165/properties-of-aromatic-compounds-635-3608/
- http://hyperphysics.phy-astr.gsu.edu/hbase/Organic/Aromatic.html